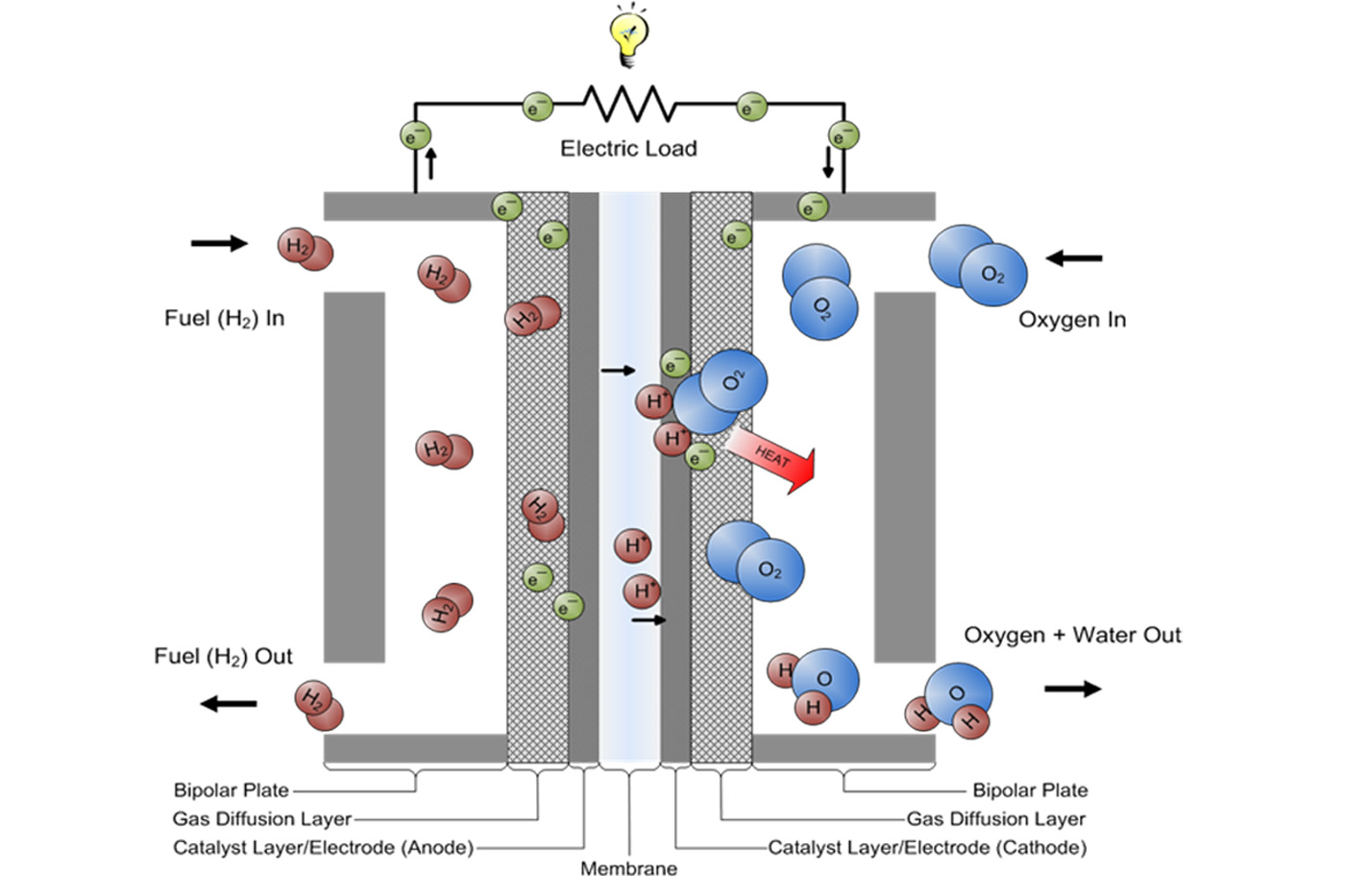
A Simple Fuel Cell Combines Hydrogen And Oxygen To Form . a hydrogen fuel cell essentially consumes hydrogen and oxygen. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a chemical cell produces a voltage until one of the reactants is used up. Fuel cells are used today in a range of applications, from. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. Water is the only product. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte.
from www.change-florida.com
a hydrogen fuel cell essentially consumes hydrogen and oxygen. Fuel cells are used today in a range of applications, from. Water is the only product. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a chemical cell produces a voltage until one of the reactants is used up.
Hydrogen Fuel Cells
A Simple Fuel Cell Combines Hydrogen And Oxygen To Form When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. Fuel cells are used today in a range of applications, from. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. Water is the only product. a chemical cell produces a voltage until one of the reactants is used up. a hydrogen fuel cell essentially consumes hydrogen and oxygen. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.
From www.qoncious.com
What is a Hydrogen Fuel Cell and How It Works? A Simple Fuel Cell Combines Hydrogen And Oxygen To Form in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Water is the only product. Fuel cells are used today in a range of applications, from. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. When a fuel cell is continuously supplied. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.alamy.com
Fuel cell in a laboratory. This fuel cell combines hydrogen and oxygen A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Water is the only product. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a hydrogen fuel cell essentially consumes hydrogen and oxygen. a fuel cell. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.teachengineering.org
HydrogenOxygen Reaction Lab Activity TeachEngineering A Simple Fuel Cell Combines Hydrogen And Oxygen To Form When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a chemical cell produces a voltage until one of the reactants is used up. a hydrogen fuel cell essentially consumes hydrogen and oxygen. Fuel cells are used today. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From frpc.net.technion.ac.il
Hydrogen and Electric Energy Production and Storage, Fuel Cells A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Fuel cells are used today in a range of applications, from. Water is the only product. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. a hydrogen fuel cell. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.researchgate.net
Schematic illustration of a hydrogen fuel cell. Download Scientific A Simple Fuel Cell Combines Hydrogen And Oxygen To Form a hydrogen fuel cell essentially consumes hydrogen and oxygen. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a chemical cell produces a voltage until one of the reactants is used up. a fuel cell like this will continue to operate and produce electrical energy as long as a supply. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From greenfuture.io
What Are Hydrogen Fuel Cell Cars? A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Water is the only product. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cells are used today in a range of applications, from. a fuel cell consists of two electrodes—a negative electrode (or anode) and a. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.teachoo.com
Hydrogen and oxygen combine in the ratio of 18 by mass to form water A Simple Fuel Cell Combines Hydrogen And Oxygen To Form a chemical cell produces a voltage until one of the reactants is used up. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. a hydrogen fuel cell essentially consumes hydrogen and oxygen. Water is the only product. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water.. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.change-florida.com
Hydrogen Fuel Cells A Simple Fuel Cell Combines Hydrogen And Oxygen To Form in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 A Simple Fuel Cell Combines Hydrogen And Oxygen To Form When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. a chemical cell produces a voltage until one of the reactants is used up. Fuel cells are used today in a range of applications, from. Water is the only product. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.yourelectricalguide.com
Fuel Cell Working Principle your electrical guide A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Fuel cells are used today in a range of applications, from. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a hydrogen fuel cell essentially consumes hydrogen and oxygen. Water is the only product. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. a chemical cell. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From mmerevise.co.uk
Commercial Applications of Electrochemical Cells MME A Simple Fuel Cell Combines Hydrogen And Oxygen To Form in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a chemical cell produces a voltage until one of the reactants is used up. a fuel cell like this will continue. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.designlife-cycle.com
Hydrogen Fuel Cell — Design LifeCycle A Simple Fuel Cell Combines Hydrogen And Oxygen To Form in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a hydrogen fuel cell essentially consumes hydrogen and oxygen. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. Fuel cells are used today in a range of applications, from. Water is the only product. a fuel cell. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From exolmxiop.blob.core.windows.net
Hydrogen Fuel Cells Generate Electricity By Combining Hydrogen With A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Fuel cells are used today in a range of applications, from. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a hydrogen fuel cell essentially consumes hydrogen. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Fuel cells are used today in a range of applications, from. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. Water is the only product. a hydrogen fuel cell essentially consumes hydrogen and. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle A Simple Fuel Cell Combines Hydrogen And Oxygen To Form a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a hydrogen fuel cell essentially consumes hydrogen and oxygen. Water is the only product. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. a chemical cell produces a voltage until. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.shalom-education.com
Fuel Cells GCSE Chemistry Revision A Simple Fuel Cell Combines Hydrogen And Oxygen To Form a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells are used today in a range of applications, from. Water is the only product. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.simscale.com
Hydrogen Fuel Cell Simulation & Modeling Blog SimScale A Simple Fuel Cell Combines Hydrogen And Oxygen To Form Water is the only product. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product. a chemical cell produces a voltage until one of the reactants is used up. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cells are used today in a range of applications,. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the A Simple Fuel Cell Combines Hydrogen And Oxygen To Form a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a chemical cell produces a voltage until one of the reactants is used up. in a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cells are used today in a range. A Simple Fuel Cell Combines Hydrogen And Oxygen To Form.